A molarity calculator is a tool that is used to convert the mass concentration of any solution against the molar concentration. It can also be used to recalculate the number of grams against the number of moles of a solution. By using a molarity calculator, the mass of a substance that needs to achieve the desired molarity can also be calculated. In simpler terms, molarity expresses the concentration of a solution and is, therefore, the number of moles of a substance that is dissolved in a solution. Molarity calculators are found online and used by chemistry students, teachers, and other practitioners in the field of science.
Molarity
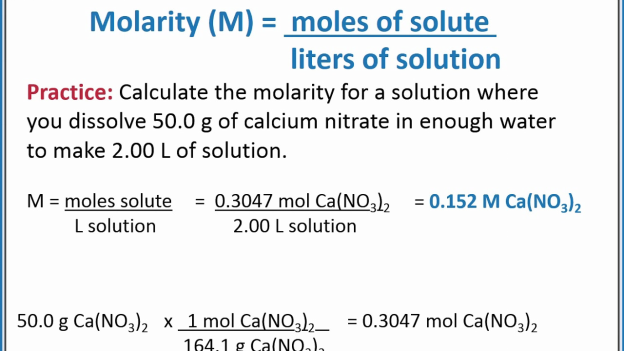
Molarity is the number of moles or the concentration in a substance or a solute.
What is a mole?
A mole is an SI unit of measurement for the amount of substance.
The difference between a mole and molarity is that a mole represents the quantity of material, while the term molarity refers to the concentration of the said material in a substance.
How is molarity calculated?
The formula for calculating molarity is (concentration/molar mass)
Concentration- the mass concentration of the solution. Concentration is expressed in grams per liter of grams per milliliter.
Molar mass- This is the constant property of a substance. It is the mass of one mole of the solute. The molar mass is expressed in terms of grams per mole.
Another formula that can be used to calculate molarity is; Moles of a solute/liters of the solution

Calculating the number of moles
Different elements have a different number of moles. In most cases, molecules in a substance are made up of more than one element. Therefore, to determine the exact molar mass of a molecule, you have to identify the elements that are present in the molecule first. Secondly, a periodic table will be instrumental when determining the molar mass for every element. Once you have the molar mass within every element, you can add them up to come up with the molar mass of the entire molecule.
If the molecules of the solute are presented in grams, you then have to convert the number of grams into moles before calculating the molarity. The formula here is (1 mole/molar mass). With moles number, you can now calculate the molarity effortlessly using the method given above.
In case the quantity of the solution is provided in milliliters, you have to convert the milliliters into liters before calculating the molarity. To do this, you divide the milliliters by one thousand.
Applications of molarity
- Molarity is used to tell the amount of a chemical that is present or dissolved in a solution
- Molarity is used in the process of understanding a chemical reaction
- It is applied in reaction balancing and balancing of molecular weight in chemistry.
Take Away
Online molecular calculators are easy tools for calculating molarity fast and accurately. Anyone can use them as they are straightforward and most of these online tools are also free